

OAN’s Brooke Mallory
4:53 PM – Thursday, January 25, 2024
The consumer health company Haleon said on Wednesday that some Robitussin products, which are promoted for the treatment of cough, flu, and sore throat symptoms, are being recalled nationally due to a microbiological contamination.
Advertisement
As the cold and flu season is rapidly approaching, the recall comes at the worst time.
Eight lots of Robitussin Honey CF Max Day and Max Night cough syrup in four- and eight-ounce bottles are affected by the recall, and the items that have been recalled are “set to expire in May 2025 or June 2026.”
According to the FDA press release, immunocompromised patients may be susceptible to the possible contamination, which could result in serious or even fatal outcomes such as fungemia, blood fungal infections, or disseminated fungal infections.
Nonetheless, individuals who are not immunocompromised are most likely not at risk of developing any of the above life-threatening conditions. However, according to the FDA, “the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
This recall covers only the following lots:
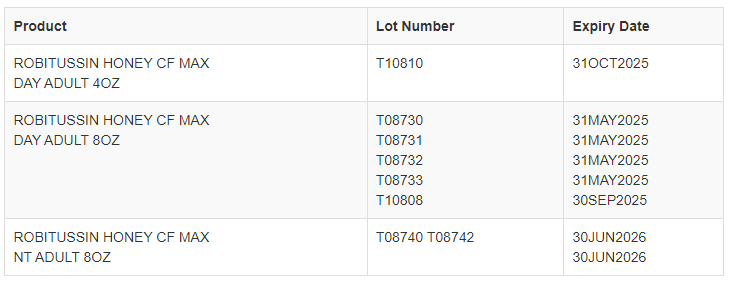

“Consumer safety and product quality are our utmost priorities at Haleon,” the company said in a statement to ABC News on Thursday. “After a thorough investigation, a root cause has been identified, and we are implementing corrective and preventative actions to ensure that this does not recur.”
Stay informed! Receive breaking news blasts directly to your inbox for free. Subscribe here. https://www.oann.com/alerts

