
OAN’s Abril Elfi
11:50 AM – Monday, July 17, 2023
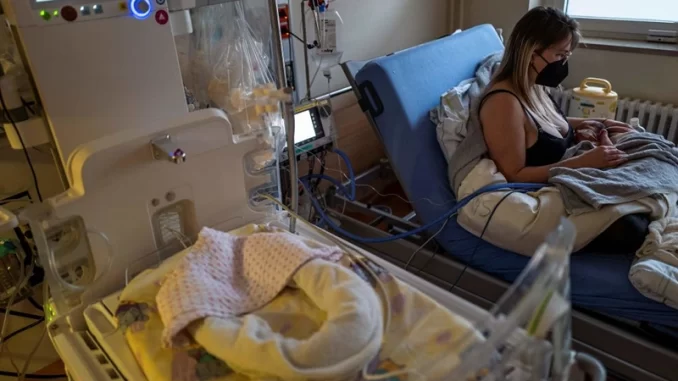
The Food and Drug Administration has approved a monoclonal antibody for protecting newborns and young children against serious RSV disease, on Monday.
Advertisement
Parents and pediatricians will reportedly have the ability to protect infants from the lung-attacking virus using the antibody treatment Nirsevimab, as soon as this fall, which will be sold under the name Beyfortus.
RSV is a virus that affects people of all ages and causes acute respiratory infections. While the majority of babies and young children only have mild, cold-like symptoms, some infants, especially with their first infection, go on to develop lower respiratory tract diseases like pneumonia and bronchiolitis. The most vulnerable groups to developing severe RSV disease are premature infants who have major congenital heart disease or chronic lung disease.
RSV is believed to lead around 2.1 million children under the age of 5 to seek outpatient care in the United States each year, and between 58,000 and 80,000 kids in that age range are hospitalized as a result of these illnesses. RSV infections are estimated to be the cause of between 100 and 300 child fatalities each year in the U.S., while being the second most common cause of infant deaths worldwide (behind malaria).
“RSV can cause serious disease in infants and some children and results in a large number of emergency department and physician office visits each year,” said John Farley, M.D., M.P.H., director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “Today’s approval addresses the great need for products to help reduce the impact of RSV disease on children, families and the health care system.”
When a baby is delivered during the respiratory syncytial virus season, which normally lasts from November to late March, the antibodies are administered in a single injection at or shortly after birth. The injection would be administered to newborns who were born at various times of the year in the first fall. Additionally, injections would be given to children at a high risk of getting serious sickness from RSV infection in order to help protect them during their second RSV season.
“Beyfortus represents an opportunity for a paradigm-shift in preventing serious respiratory disease due to RSV across a broad infant population in the U.S.,” Iskra Reic, AstraZeneca’s executive vice president for vaccines and immune therapies, said in a statement.
Beyfortus has antibodies that have already developed and can immediately defend infants from serious illness. According to trials done on adults, the antibodies were seen in the blood of recipients within hours of administration and peaked by day six.
According to the FDA, Rash and injection site responses are two possible Beyfortus side effects. Infants and kids having a history of severe hypersensitivity responses to Beyfortus’ active ingredients or any of its excipients shouldn’t be administered the medication.
Canada, the United Kingdom, and the European Union have already granted Beyfortus licenses. Sanofi will market it in the U.S. after AstraZeneca developed it.
The Centers for Disease Control and Prevention’s independent expert advisory committee, the Advisory Committee on Immunization Practices, still needs to endorse the use of Beyfortus. The ACIP has scheduled a special meeting for August 3rd to decide on whether to recommend the antibody and whether to include it in the Vaccines for Children program, necessitating that the federal government cover the cost of it for kids without insurance.
This fall, there may be more ways to prevent the illness besides Beyfortus. The FDA is debating whether to approve Pfizer’s vaccination for expectant mothers, which also protects unborn children. In that situation, the mother produces the antibodies, which pass through the placenta to protect the fetus and are predicted to endure for the first few months of an infant’s life.
Stay informed! Receive breaking news blasts directly to your inbox for free. Subscribe here. https://www.oann.com/alerts

